We are excited to announce that RegDesk, the innovative Regulatory Information Management (RIM) platform, has been recognized as a Representative Vendor in the Gartner® Market Guide for Life Sciences Regulatory Information Management. Read the complimentary Market Guide for RIM Solutions report to learn more!
Table of Content
The Gartner Market Guide provides a holistic framework for understanding the maturity, adoption, and potential impact of emerging technologies. We feel inclusion in this prestigious report signifies that RegDesk is positioned to become a mainstream solution within the RIM/ Medtech space.
The major takeaway from the Gartner Market Guide emphasizes the growing need for RIM solutions to provide the framework to manage all aspects of the regulatory submission process. As stated by Gartner, “Life science CIOs want to reduce the complexity of their regulatory IT systems and accompanying traditional business processes to speed regulatory submissions and lower costs. Vendors that focus on end-to-end regulatory information management (RIM) address these challenges by reducing unnecessary pre production work, quality checks and complex system integrations via middleware solutions.”
RegDesk addresses this need perfectly, offering a comprehensive suite of features powered by cutting edge AI and automation. Regdesk’s centralized platform maximizes global team efficiency by streamlining workflows and eliminating hours wasted on search engines, spreadsheets and silos.
Key Features of RegDesk RIM Platform:
- AI Submission Generator: Streamline your submission process with our advanced AI powered assistance. Craft comprehensive, compliant submissions in hours, not months, utilizing country specific templates tailored to meet local regulations. Say goodbye to tedious manual work and focus on strategic tasks that matter.
- AI Form Builder: Simplify complex form completion with our intelligent automation. Easily fill out lengthy documents such as the General Safety and Performance Requirements (GSPR) and Declaration of Conformity (DoC). Built in intelligence ensures accuracy and saves you time, reducing the risk of errors and rework.
- Regulatory Intelligence: Stay ahead in the ever evolving regulatory landscape with daily updates and alerts. Utilize comprehensive checklists and access a vast database of global regulations covering over 120 countries. Be proactive, not reactive, with insights that keep you informed and prepared for every regulatory shift.
- Global Application Management: Manage your regulatory submissions seamlessly across the globe. Track every step of your applications in real time, coordinate effortlessly with international teams, and ensure compliance with diverse regulatory requirements. Achieve smooth, coordinated processes no matter where your teams are located.
- Distributor Collaboration Tools: Enhance your partnership with distributors and stakeholders worldwide. Our platform allows you to connect efficiently, share documents securely, and maintain control over international submissions. Streamline your workflow and keep your collaboration focused and productive.
- Change Control Projects: Transform your change management with intelligent automation and regulatory insight. Automate change assessments to quickly identify compliance implications, utilize regulatory intelligence to make informed decisions, and streamline collaboration with both internal teams and external partners. Stay agile and responsive to regulatory changes without the chaos of manual processes.
- Standards Management: Stay ahead of industry requirements with instant access to a vast library of over 200,000 standards. Receive automatic updates on any changes to standardsand effortlessly conduct impact assessments to understand their effects on your operations. Simplify compliance management and ensure your organization is always aligned with the latest industry standards.
RegDesk: The Powerhouse of Holistic RIM
RegDesk revolutionizes traditional RIM with an integrated, AI powered suite. This holistic platform empowers life sciences companies to enhance compliance and efficiency. With our forward thinking RIM vision, we empower life sciences companies to enhance compliance and efficiency. As per Gartner “The availability of new technologies such as artificial intelligence (AI), robotic process automation (RPA) and low-code platforms are driving new investments in, and increasing expectations of, RIM solutions.”
Complimentary Access to this Report Here
Beyond Efficiency: A Commitment to Continuous Innovation
Experience the future of regulatory compliance with RegDesk. As the ONLY RIM platform offering integrated regulatory intelligence and automated workflows, RegDesk empowers your regulatory team to efficiently prepare, manage, and track global submissions and seamlessly perform assessments.
Clients praise our platform for enhancing visibility across global regulatory teams, automating processes to eliminate rework, and substantially reducing the time and cost associated with international submissions. Committed to continuous innovation, RegDesk focuses on digital technologies, intelligence, and integration. We are relentlessly advancing our platform with new features to stay ahead of evolving regulatory needs.
Discover how RegDesk can transform your regulatory function and drive your business forward. Join us on the journey to regulatory excellence.
Request a free demo today and experience the future of RIM
Gartner, Market Guide for Life Science Regulatory Information Management Solutions, Jeff Smith, 2 September 2024.
GARTNER is a registered trademark and service mark of Gartner, Inc. and/or its affiliates in the U.S. and internationally and is used herein with permission. All rights reserved. Gartner does not endorse any vendor, product or service depicted in its research publications, and does not advise technology users to select only those vendors with the highest ratings or other designation. Gartner research publications consist of the opinions of Gartner s research organization and should not be construed as statements of fact. Gartner disclaims all warranties, expressed or implied, with respect to this research, including any warranties of merchantability or fitness for a particular purpose.
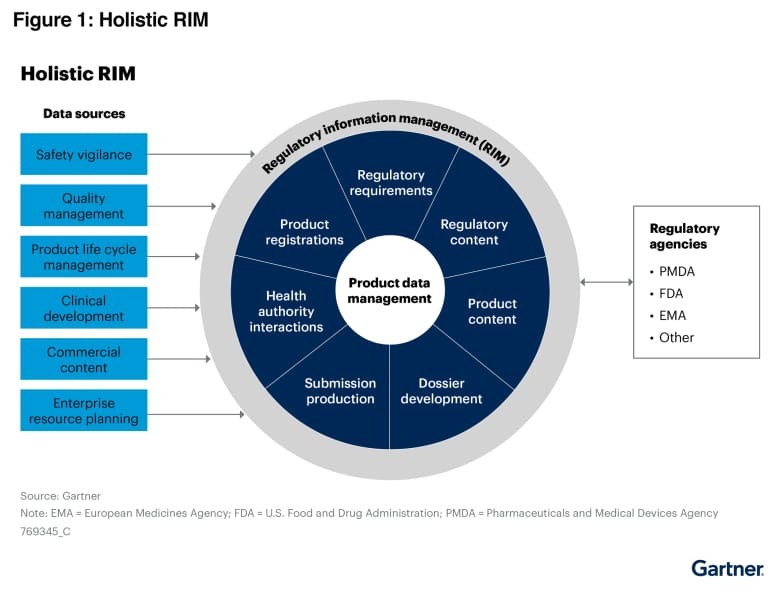
This graphic was published by Gartner, Inc. as part of a larger research document and should be evaluated in the context of the entire document.
The Gartner document is available upon request from RegDesk.

