Increased Visibility of Compliance Changes and Automation with RegDesk RIMS Triples Global Submissions and Increases Quality
Situation
A 100-year-old medical device company focusing on cardiothoracic devices with offices in 27 nations and operating globally struggled to manage the complexity of having multiple devices in multiple markets. The regulatory team was unable to keep up with the volume of work required to remain current and compliant in an ever-changing environment and avoid human errors from a very manual and cumbersome process. In addition, they struggled to collaborate across business units and track approvals in process.
Objectives
This medical device company wanted to have visibility into upcoming changes in all countries where they did business and understand how those changes would impact existing products in the field. In addition, they wanted to increase the output and accuracy of the existing team without adding headcount. These objectives were important to them so the company did not experience any lost revenue due to non-compliance.
Why They Chose RegDesk
The company selected RegDesk because of its comprehensive database of regulatory changes by country, its capability to pinpoint products requiring attention, and its ability to automate the submission process workflow. This enhanced visibility would help the team minimize wasted effort, reduce errors, and improve communication across business lines. Additionally, they could apply metrics to track market-level data to identify resources necessary to avoid delays in market access.
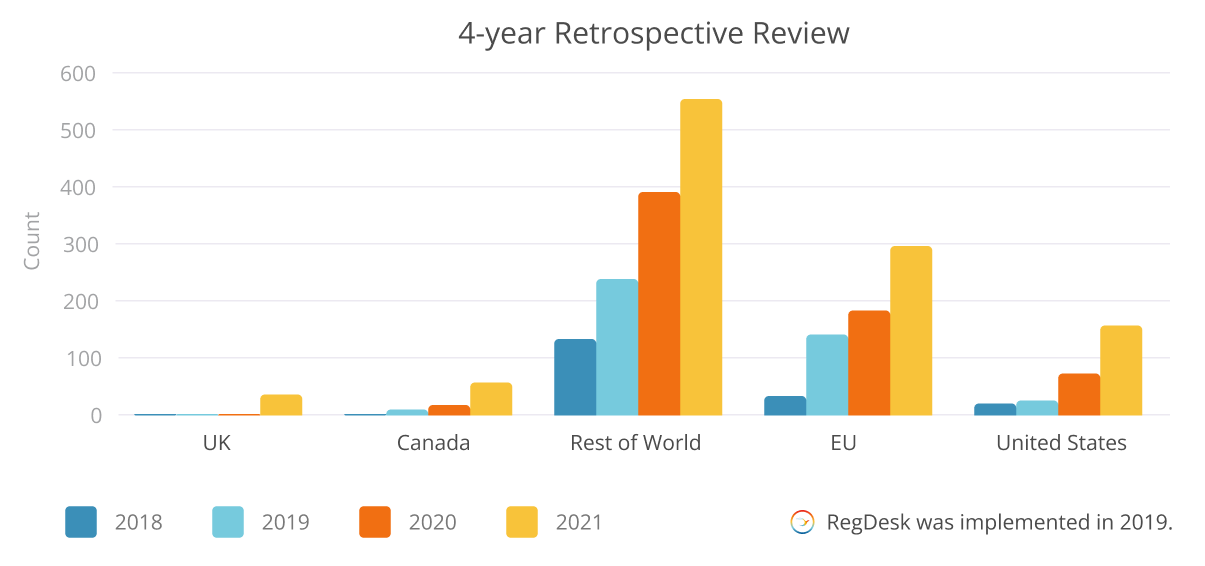
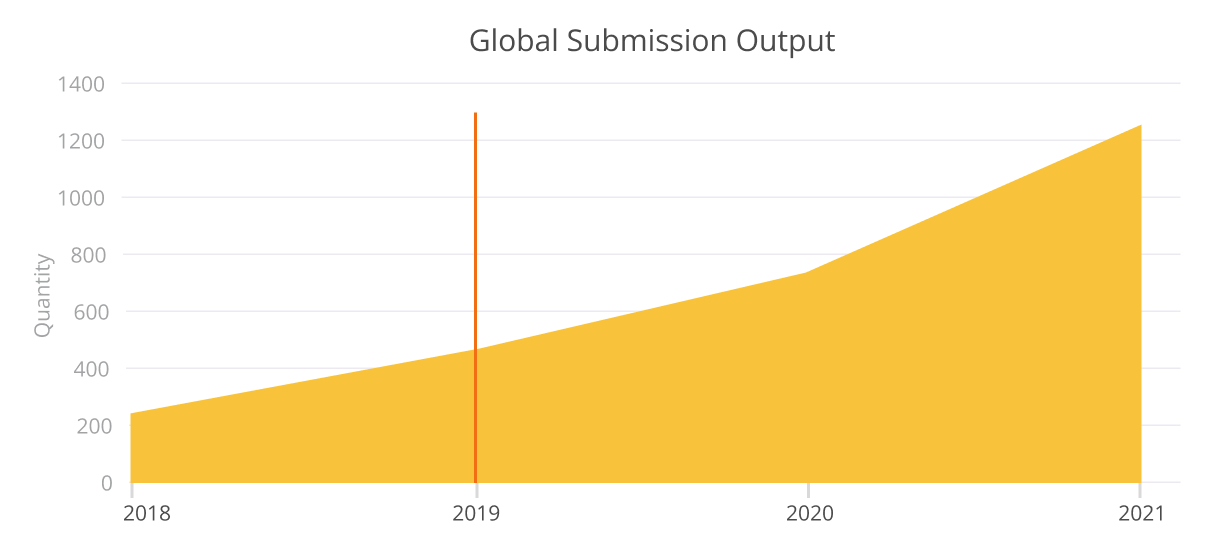
Results
The team was able to vastly improve their output and become more accurate in their submissions, which created additional capacity for the team as they moved from being reactive to being proactive in their submission process. Moreover, they achieved improved internal collaboration and streamlined reporting processes. With RegDesk, they were able to substantially elevate both the quality and quantity of their submissions.