 North America
North America
Theranos FDA Scandal: A Closer Look
The difference between CLIA and FDA standards
 North America
North America
The difference between CLIA and FDA standards
 Africa
Africa
The 1960s are a time remembered either fondly or distastefully, depending on your designated family historian. If a friend or family member happens to be a medical professional, they will note one important event in their field which lay audiences may not be entirely...
 North America
North America
Many manufacturers believe that selling their product or device without FDA approval is acceptable. If people buy it, but is this breach in ethics worth the potential consequences? If caught marketing an unapproved product, the FDA may issue a warning letter...
 North America
North America
As the patents for current biologics expire and the biosimilar industry begins to gain traction it is important to look at the future of the biosimilar market while noting hold-backs within the US manufacturing and approval processes. According to Research and...
 North America
North America
The FDA has announced an increase in fees for assessing and exporting medical devices. The FDA Export Reform and Enhancement Act of 1996 enables manufacturers to request that the FDA issue a certification stating that their device meets certain requirements within 20...
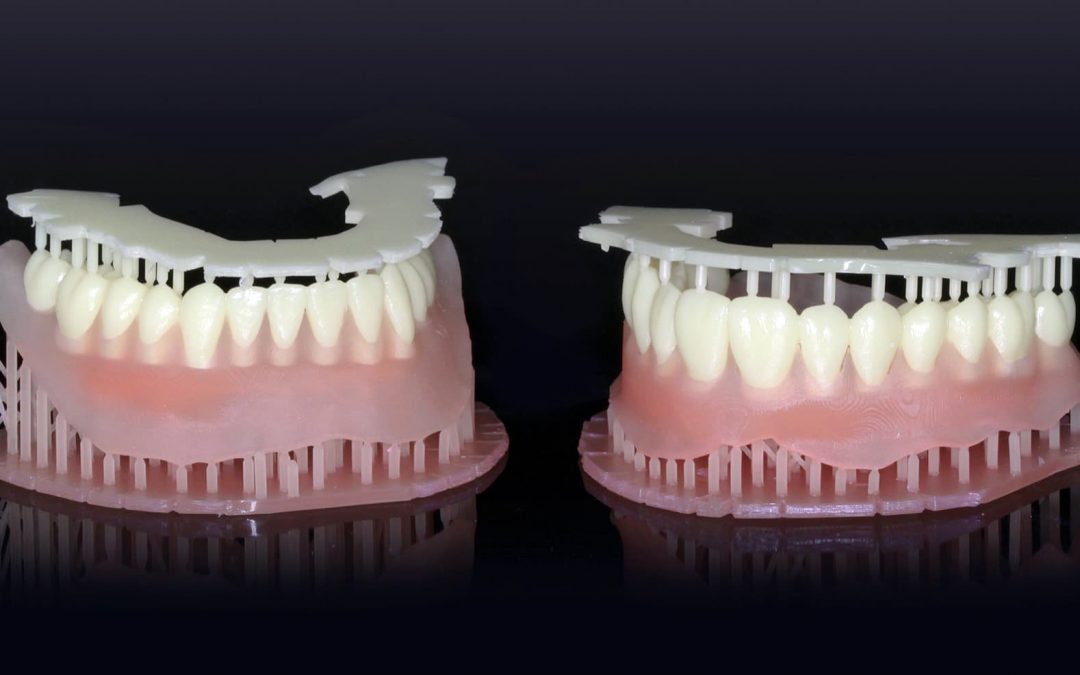 North America
North America
Dentca, a dental laboratory, received its first clearance from the FDA for a 3D printed resin that can be used as a base material during the manufacturing process for dentures. This is a huge step in the regulatory world because it is accepting 3D printing as an...