Over the last two decades, biologics have revolutionized the treatment of illnesses such as diabetes, cancer, rheumatoid arthritis and hepatitis. However, many prominent biologics are about to have their patents expire in the U.S., making way for biosimilars.
Biologics:
Biologics come in the form of vaccines, allergenics, gene therapy, tissues or recombinant therapeutic proteins. Many biologics are:
- Composed of sugars, proteins, nucleic acids, a combo of all three, naturally produced enzymes or monoclonal antibodies
- Derived from living things such as cells, tissues or hormones and produced by biotechnology
Due to their complex nature and high demand, biologics have become very expensive to produce and develop. Since half of the biologics currently on the market are about to have their patents expire, biosimilars are gaining the opportunity to enter the field.
Biosimilars
Biosimilar regulations are mostly known as generic copies of already approved biologics. They are usually manufactured by a different company than the innovator and can only be marketed when the original patent is up.
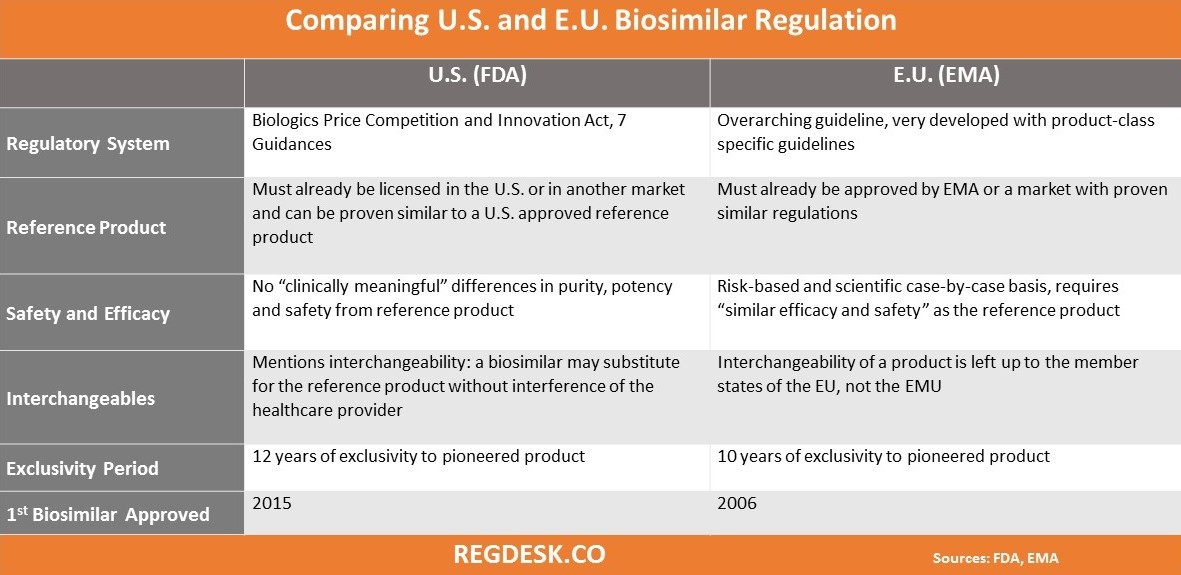
The FDA has only recently adapted regulations on biosimilars similar to that in the E.U. Because the EMA established a regulatory process of approval for biosimilars years before the FDA, the E.U. currently has 19 approved biosimilars while the U.S. has only one. The following chart is an overview of six standing differences between FDA and EMA biosimilar regulations: