The European Commission’s Health Technology Assessment Regulation (HTAR) marks a significant step toward harmonizing health technology evaluations across EU member states. Effective from January 2025, this regulation aims to enhance innovation, streamline decision-making processes, and improve patient access to cutting-edge healthcare solutions.
Key updates include the establishment of the HTA Coordination Group, joint clinical assessments, and new stakeholder engagement strategies. Stay informed as the European Union (EU) prepares for this transformative healthcare milestone.
Table of Content
Overview of HTAR and its Significance
The Health Technology Assessment Regulation (HTAR) is a critical EU framework that standardizes health technology evaluations, such as assessments for medicines and medical devices, across member states. It fosters greater collaboration among countries to conduct joint clinical assessments (JCAs) and consultations, ensuring more efficient decision-making and reducing duplicative efforts. Where the EU MDR ensures a medical device is safe and effective for market entry, HTAR evaluates clinical effectiveness and impact.
By providing high-quality evaluations, HTAR supports innovation while improving patient access to cutting-edge treatments. This initiative plays a key role in shaping a more efficient and equitable healthcare system across the European Union (EU).
Key Dates: Entry into Force and Applicability
The Health Technology Assessment Regulation (HTAR) represents a transformative step in EU healthcare policy. It officially entered into force on January 11, 2022, setting the foundation for collaborative health technology evaluations across member states.
The regulation becomes fully applicable on January 12, 2025, marking the start of its practical implementation. This transitional phase allows member states and key stakeholders to adapt their systems and processes to meet new requirements, including joint clinical assessments and scientific consultations.
The duration of the transitional phase for the regulation effective January 12, 2025, varies depending on the specific regulation in question. For example, markets in Crypto-Assets (MiCA) Regulation provides a maximum transitional period of 18 months for Virtual Asset Service Providers (VASPs).
However, other EU member states have adopted shorter transitional periods ranging from 5 to 12 months. These developments aim to enhance efficiency, innovation, and patient access to advanced health technologies.
Key Implementation Updates
The implementation of the Health Technology Assessment Regulation (HTAR) has seen substantial progress, with the European Commission establishing frameworks to ensure smooth adoption across EU member states. The creation of the Health Technology Assessment Coordination Group (HTACG) plays a vital role in facilitating joint clinical assessments and scientific consultations.
Key Implementation Key Points
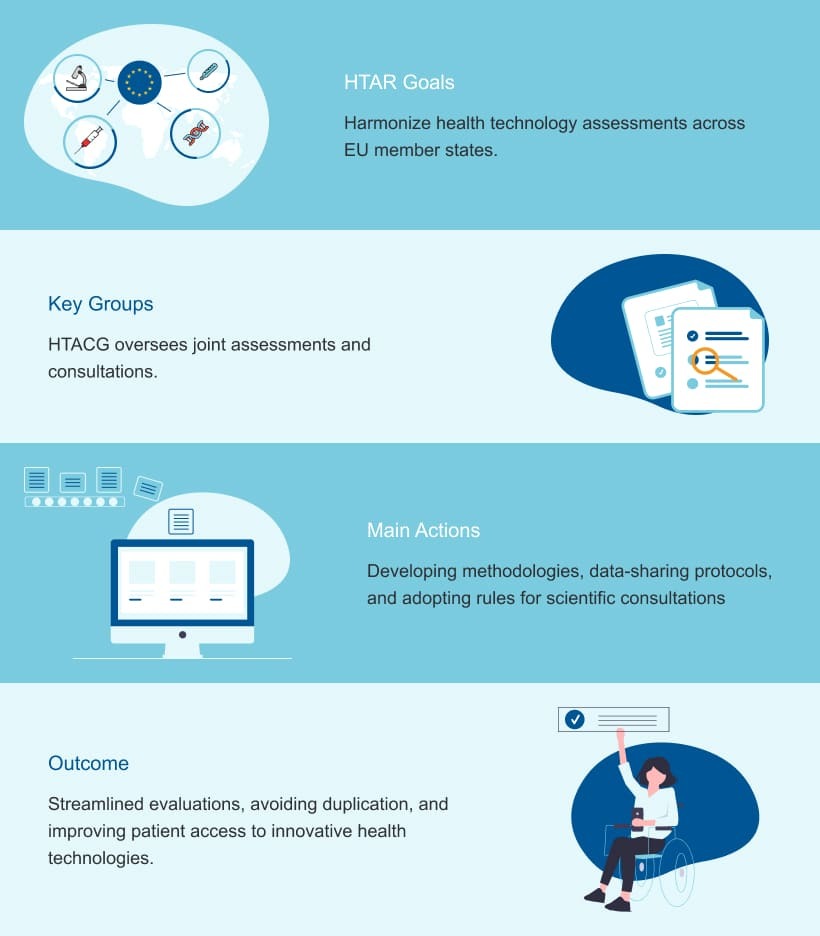
Progress on the Implementation Rolling Plan
The progress on the implementation rolling plan for the Health Technology Assessment Regulation (HTAR) is a key aspect of ensuring the regulation’s smooth rollout across the EU. This plan outlines a series of coordinated steps to prepare all involved stakeholders for full implementation by 2025.
Significant developments include the creation of tools to support joint clinical assessments and scientific consultations, as well as enhancing collaboration among EU member states. Ongoing updates to the plan allow for adjustments based on emerging challenges and ensure that all timelines are met.
These efforts will streamline health technology evaluations and foster more efficient and transparent processes across Europe.
Role and Recent Activities of the HTA Coordination Group (HTACG)
The Health Technology Assessment Coordination Group (HTACG) is crucial to the smooth implementation of the HTAR. It ensures that EU member states work together effectively in assessing health technologies, facilitating the exchange of knowledge, and aligning methodologies.
Recently, HTACG has focused on refining the technical infrastructure, such as data-sharing platforms and the development of common assessment methodologies, to streamline evaluations. This collaboration aimed to ensure that the HTAR was fully operational by January 2025, enhancing healthcare decision-making across Europe. This collaboration became fully applicable on January 12, 2025.
Joint Clinical Assessments (JCA) and Scientific Consultations (JSC)
Joint Clinical Assessments (JCAs) and Joint Scientific Consultations (JSCs) are essential components of the Health Technology Assessment Regulation (HTAR), promoting collaboration across EU member states. JCAs enable a unified, evidence-based approach to evaluating the clinical effectiveness of new health technologies, reducing duplication of efforts and ensuring a consistent standard of assessment.
How JCAs and JSCs Shape Technology in the EU
Key Benefits
Reduced duplication of clinical assessments
Early-stage guidance for technology developers
Transparent and evidence-based decision-making
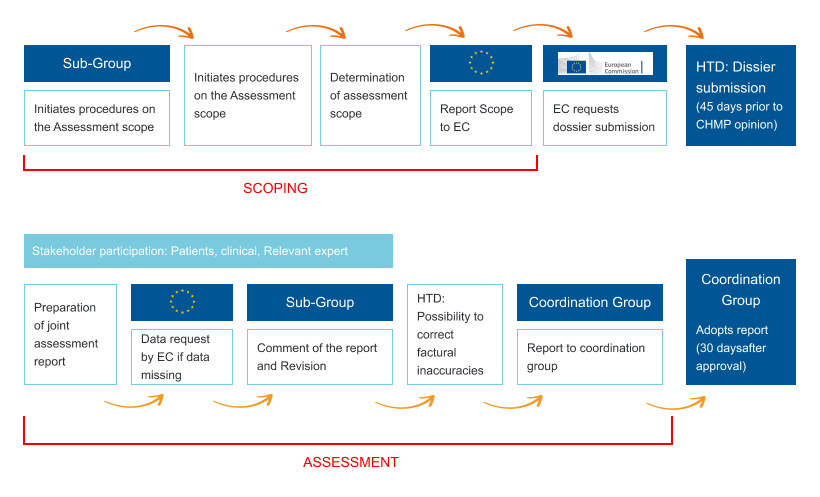
How it Works
Joint Clinical Assessments (JCA) and Scientific Consultations (JSC)
Joint Clinical Assessments (JCAs) and Joint Scientific Consultations (JSCs) are essential components of the Health Technology Assessment Regulation (HTAR), promoting collaboration across EU member states. JCAs enable a unified, evidence-based approach to evaluating the clinical effectiveness of new health technologies, reducing duplication of efforts and ensuring a consistent standard of assessment.
Impact
More efficient healthcare innovation, quicker patient access, and higher standards across the EU. JSCs offer a platform for early scientific discussions between health technology developers and regulatory bodies, providing guidance on clinical development and regulatory strategies.
Together, these processes support more efficient, transparent, and streamlined healthcare decision-making in the EU. The framework for Joint Clinical Assessments (JCAs) under HTAR establishes consistent methodologies and criteria to assess the clinical effectiveness, safety, and impact of health technologies.
These assessments are based on rigorous evidence from clinical trials and real-world data, ensuring objective, standardized evaluations. The process involves collaboration between the EU member states and experts, fostering transparency and improving decision-making.
The goal is to accelerate access to innovative health technologies while maintaining high safety and efficacy standards. Recent consultations and engagement opportunities regarding the Health Technology Assessment Regulation (HTAR) have focused on gathering stakeholder input for its effective implementation.
These consultations provided a platform for health technology developers, patient groups, and other stakeholders to offer feedback on assessment methodologies and scientific consultation processes.
Key Stages of the Health Technology Assessment Process
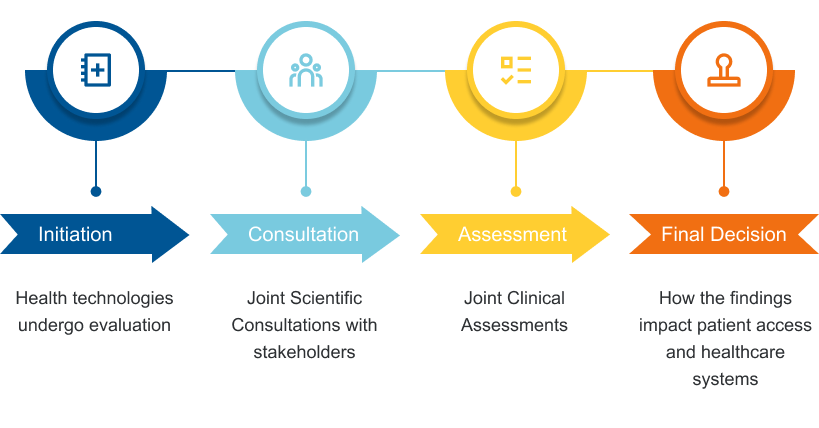
Latest Developments
Recent developments regarding the Health Technology Assessment regulation (HTAR) reflect significant progress in streamlining health technology evaluations across the European Union (EU). The HTAR aims to reduce duplication in assessments by promoting cooperation between member states and ensuring more timely and transparent evaluations of health technologies, such as medicines and medical devices.
As part of these updates, the regulation introduces joint clinical assessments (JCAs) and joint scientific consultations (JSCs), facilitating collaboration between member states and allowing for a more unified approach to evaluating the clinical effectiveness and safety of health technologies. These efforts are expected to foster a more efficient and equitable healthcare system across Europe, improving patient access to the latest treatments and innovations.
Furthermore, the ongoing engagement of various stakeholders, including health professionals, patient organizations, and technology developers, has been crucial in refining the HTAR framework. Recent consultations and workshops have allowed these groups to provide valuable feedback on the regulation’s implementation, helping to identify areas for improvement and further collaboration.
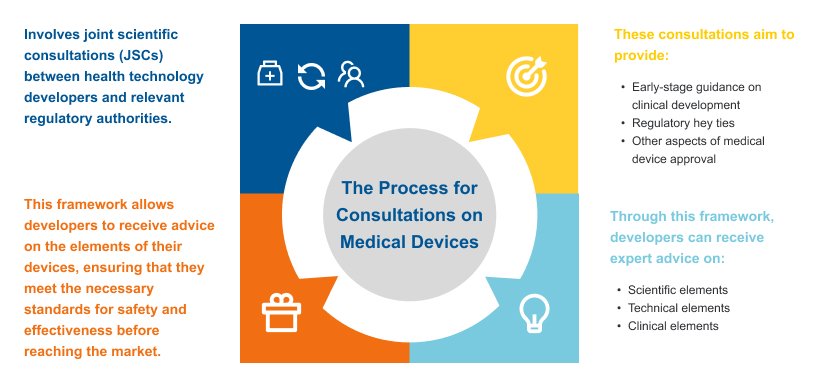
Adoption of Rules for JCAs and Consultations on Medical Devices
The adoption of rules for Joint Clinical Assessments (JCAs) and consultations on medical devices under the Health Technology Assessment Regulation (HTAR) marks a significant step toward ensuring consistent and efficient evaluations across the EU. These rules are designed to harmonize the assessment process for health technologies, including medicines and medical devices, across member states.
By establishing common methodologies and guidelines for conducting JCAs, the regulation facilitates a unified approach to evaluating the clinical effectiveness and safety of medical devices.
Resources for Further Information and Updates
For those seeking further information and updates regarding the Health Technology Assessment Regulation (HTAR), the European Commission provides a dedicated page that includes detailed information on the regulation’s implementation, key milestones, and the latest developments. This page serves as a central hub for resources related to HTAR, offering access to the implementation rolling plan, reports, and other important documents.
Conclusion
The Health Technology Assessment Regulation (HTAR) represents a significant step forward in creating a more collaborative and efficient framework for evaluating health technologies across the European Union (EU). The regulation aims to streamline assessments, reduce duplication, and ensure timely access to innovative therapies for patients.
With its focus on joint critical assessments and scientific consultations, the HTAR fosters greater cooperation among member states, ultimately improving the quality and consistency of health technology evaluations. The resources provided by the European Commission, including the rolling implementation plan and regular updates, ensure that stakeholders are informed and prepared for the full implementation of the regulation.
The Health Technology Assessment Regulation (HTAR) became applicable across the European Union beginning January 12, 2025. From this date, joint clinical assessments will be conducted for certain medicinal products, including those with new active substances indicated for cancer treatment and advanced therapy medicinal products.

