The Food and Drug Administration (FDA), the US regulating authority in the sphere of medical devices and other healthcare products, announced the new improvements and amendments to the Software Precertification Pilot Program.

FDA Pre-Cert Pilot Program in Brief
According to the official information published by the FDA, the Software Precertification (Pre-Cert) Pilot Program is a special project aimed at the future development of the regulatory framework for medical software. In particular, the FDA intends to accelerate the regulatory procedures to be performed before placing the Software as a Medical Device (SaMD) on the market while ensuring it is fully compliant with the applicable safety and performance requirements. The new framework should also reduce the unneeded regulatory burden carried by both medical device manufacturers and the FDA. The authority emphasizes that in order to meet the applicable criteria, the manufacturer (developer) of software with the intended medical purpose shall demonstrate a robust culture of quality and organizational excellence. The manufacturer is also obliged to establish and maintain a system of continuous monitoring of the actual performance of the software products made available to the consumers (including healthcare professionals and patients).
Thus, in accordance with the regulatory approach introduced by the Pre-Cert Pilot Program, the FDA will pay attention not only to the SaMD itself but also to the way the manufacturer builds its internal processes. The Agency states that under the new framework the factors associated with the manufacturers are even more important than ones associated with the medical software in question. To meet the eligibility criteria, the medical software manufacturer (developer) should be precertified before making its product available to the customers.
Another important point relates to modifications and improvements. The FDA emphasizes that due to their nature, software products could be amended and modified within a short period of time. These features allow the medical software developers to implement necessary changes quickly in case of certain concerns on the safety and performance of medical software. According to the position of the Agency, such a quick reaction to adverse events and errors in operations of the software is important to ensure the products available on the market meet the applicable safety and performance requirements all the time.
The first iteration of the project, Pre-Cert 1.0, was launched by the FDA earlier in 2019. The initial pilot testing was limited to the manufacturers of SaMD products. Now the Agency intends to expand the scope of the pilot Pre-Cert project to cover software in a medical device (SiMD) and other software products including ones designed to be used with hardware medical devices.

Software Precertification Program: Key Points
In order to streamline the development of the Pre-Cert Pilot Program, the FDA has developed the Software Precertification Pilot Program Working Model (its current version in 1.0). The document outlines the most important features of the new project, describes the main elements of the proposed framework, and explains the way the new approach could improve existing regulatory procedures to ensure the availability of safe and efficient medical software to healthcare professionals and patients.
As it was already mentioned before, the initial testing of the Software Pre-Cert program commenced earlier in 2019 when the Agency published the appropriate Test Plan describing the approach used to evaluate the actual performance of the new regulatory framework in comparison to existing procedures.
Later in 2020, the Agency has published an Update providing the summary of the development process inching the results obtained in the course of testing, as well as the following steps.
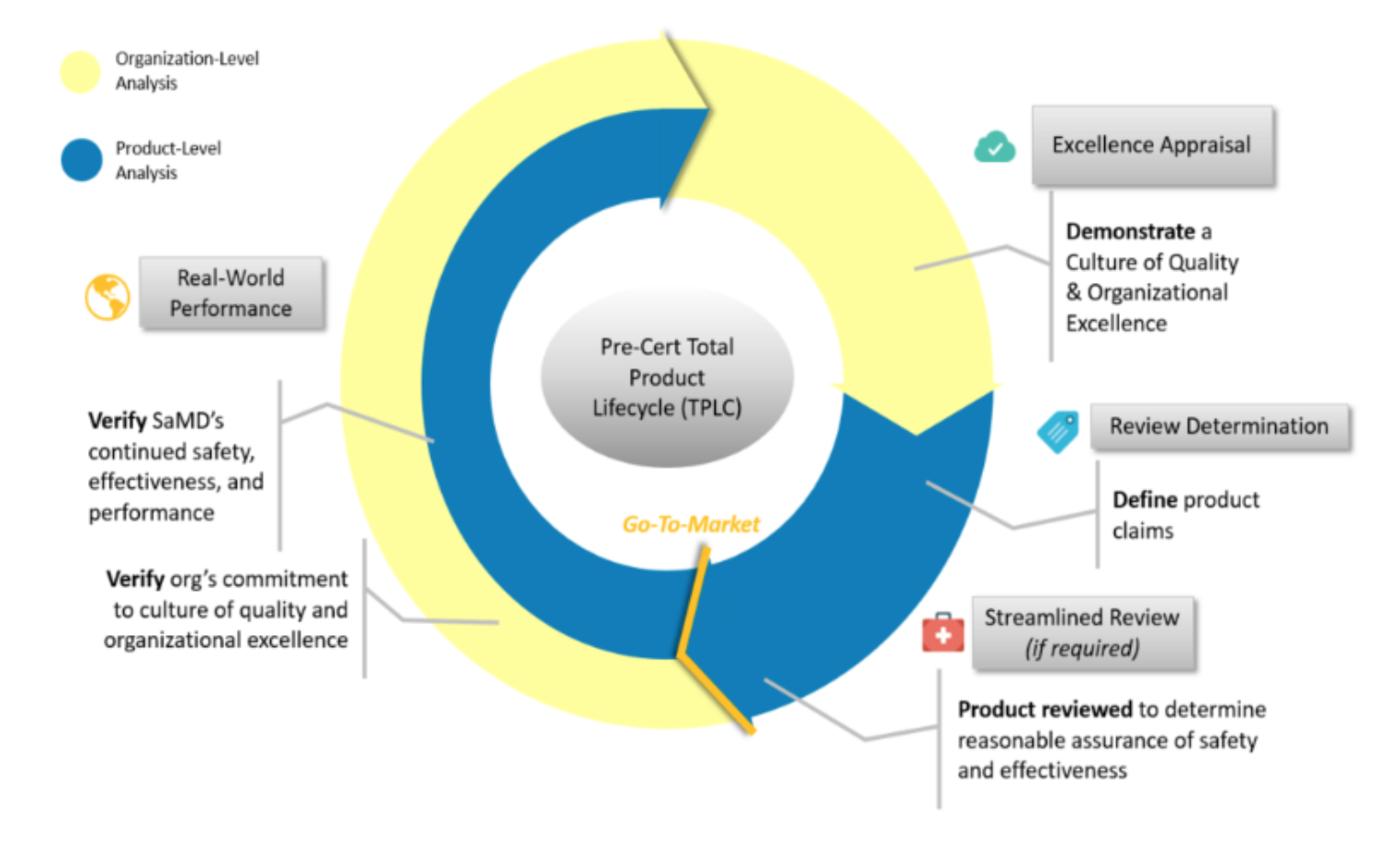
The Pre-Cert Pilot Project implements the Total Product Lifecycle Approach (TPLC) in order to establish continuous monitoring of the performance of the software at any stage of its lifecycle.
The TPLC comprises of the following key elements:
- Excellence Appraisal: determination of the special criteria to be used by the Agency when evaluating the eligibility of the medical software manufacturer (developer) for precertification. The current version of the Pre-Cert Pilot Program employs the five main criteria: patient safety, project quality, clinical responsibility, cybersecurity responsibility, and proactive culture. It is also important to mention that actually there are tow levels of precertification to be implemented: assessment of compliance with the excellence principles outlined hereabove and also the ability of the manufacturer applying for certification to provide a track record.
- Review Determination: under the Precertification Framework, the manufacturers would be allowed to determine the regulatory pathway for their products themselves. In particular, low-risk software products could be placed on the market under the simplified (notification) procedure providing that it would be sufficient just to inform the FDA about placing the software on the market, while high-risk products would be subject to the appropriate assessment and evaluation necessary to ensure that they meet the applicable safety and performance requirements. When applying this principle, the FDA is going to use the risk categorization developed by the International Medical Device Regulators Forum (IMDRF), a voluntary association of regulating authorities. The Agency will also take the steps necessary to provide healthcare professionals and patients with important information regarding the initial review and continuous monitoring activities based on the particular risk category of the medical software.
- Streamlined Review: the Agency will develop a special approach to be used when determining the scope of information to be provided by the medical software manufacturer (developer) when filing the initial submission.
- Real-world performance: according to the new approach, the medical software manufacturer shall collect and analyze the information related to the actual performance of its software products placed on the market in order to be able to identify safety concerns and adverse events, and also to make changes and modifications accordingly. At the present stage, the Agency is still considering all the options available to choose the most efficient approach.
Pre-Cert Pilot Program Purpose
According to the official announcement, the new Software Precertification Program is intended to introduce the new approach to the medical software, improve the procedures associated thereto, and to reduce the regulatory burden.
The whole concept is based on the three main points:
- transparency,
- trust, and
- verification.
The Agency also outlines the main goals of the Pre-Cert Pilot Program, namely:
- Creating benefits for precertified medical software manufacturer, allowing it to place its products on the market under the simplified and accelerated procedures,
- Improving operations with data related to the safety and performance of medical software,
- Implementation of the new approach to the changes and modifications,
- Ensuring that any and all software products allowed to be marketed in the US meet the applicable safety and performance requirements,
- Continuous improvement of the Program itself based on the information collected in the course of its application.
Summarizing the information provided here above, the update on the Pre-Cert Pilot Program development published by the FDA describes the most important aspects of the new framework and also the current status of the project. The document outlines key features of the Software Precertification approach and the main principles it is based on.
How Can RegDesk Help?
RegDesk is a next-generation web-based software for medical device and IVD companies. Our cutting-edge platform uses machine learning to provide regulatory intelligence, application preparation, submission, and approvals management globally. Our clients also have access to our network of over 4000 compliance experts worldwide to obtain verification on critical questions. Applications that normally take 6 months to prepare can now be prepared within 6 days using RegDesk Dash(TM). Global expansion has never been this simple.
Sources:

