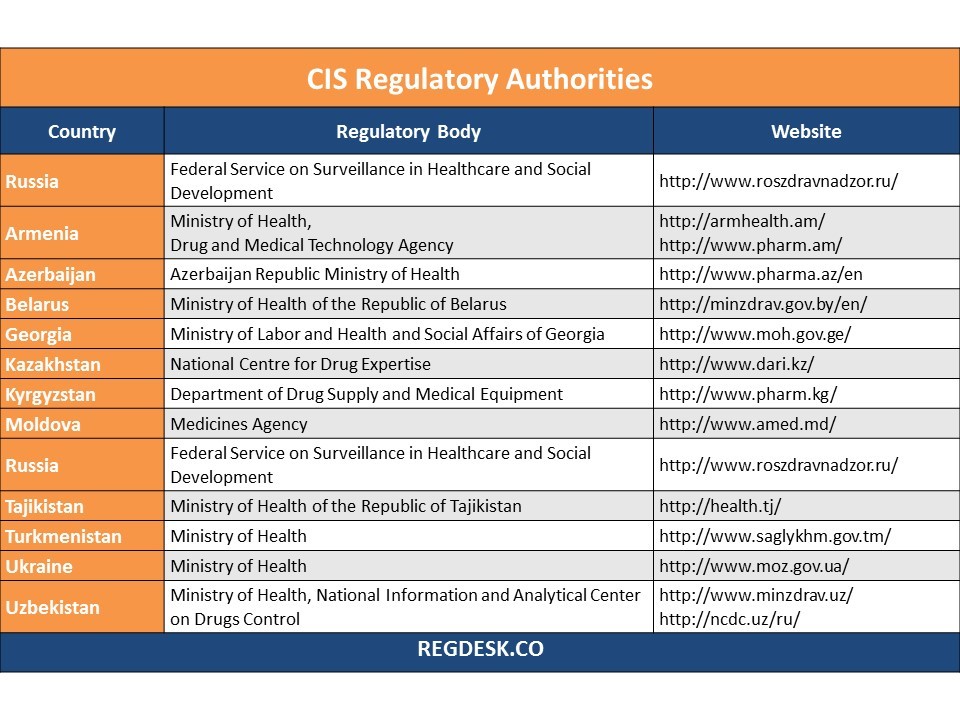
This chart lists the pharmaceutical, medical and related regulatory authorities of Commonwealth of Independent States (CIS) countries along with links to their relevant websites.
Categories
Categories
- ACTD (1)
- Africa (2)
- AI (1)
- AIaMD (1)
- ANMAT (1)
- ANVISA (1)
- Argentina (1)
- Asia (24)
- Singapore (9)
- Australia (10)
- Australia and Oceania (7)
- AusUDID (1)
- Bahrain (2)
- BfArM (4)
- Brazil (5)
- ANVISA (1)
- Canada (14)
- CBRN (1)
- CDRH (3)
- CDSCO (2)
- China (4)
- COVID-19 (1)
- DRAP (7)
- DRCPFA (1)
- EDA (2)
- Egypt (2)
- Ethiopia (2)
- EU (13)
- EU MDD (1)
- EU MDR/IVDR (18)
- EUA (1)
- Europe (58)
- European Commission (3)
- European Union (11)
- FDA (71)
- Germany (4)
- GMDN (1)
- GMLP (1)
- Great Britain (6)
- Guatemala (1)
- Health Canada (7)
- Healthcare (1)
- HIE (1)
- Hong Kong (1)
- HSA (8)
- IMDRF (1)
- India (2)
- Kazakhstan (1)
- Korea (1)
- KSA (1)
- Malaysia (2)
- MDA (2)
- MDACs (1)
- MDCG (21)
- MDR (3)
- Medical Device Regulation Resources (1)
- Medical Devices (26)
- Medsafe (2)
- Mexico (1)
- MFDS (1)
- MHRA (9)
- New Zealand (2)
- NHRA (2)
- NMPA (2)
- North America (71)
- Pakistan (7)
- PDUFA (1)
- PHEs (1)
- Philippines (1)
- Poland (1)
- RegDesk News/Info (25)
- RIMS (24)
- SAHPRA (2)
- SaMD (2)
- Saudi Arabia (12)
- Serbia (1)
- SFDA (3)
- South Africa (2)
- South America (8)
- South Korea (1)
- Swissmedic (3)
- switzerland (3)
- Taiwan (2)
- TGA (16)
- Turkey (4)
- Turkish national regulating authority (1)
- UK (7)
- Uncategorized (36)
- United Kingdom (7)
- United States (93)
- URRA (1)
- USA (1)
- USFDA (3)
Tags
medical devices (137)
RegDesk (74)
Medical Device (72)
FDA (58)
united states (30)
healthcare (29)
Healthcare Products (28)
mdr (27)
RIMS (26)
FDA guidance (25)
Regulatory Compliance (24)
regulatory (23)
guidance (23)
guidance RegDesk (23)
regulation (22)
medical device regulations (21)
Medical Devices RegDesk (19)
regulatory affairs (17)
United States of America (17)
medical software (16)
Europe (15)
medical device regulation (15)
IVDR (14)
North America (14)
medical device registration (13)
Global Manufacturers (13)
Medical Technologies (13)
EU (12)
health canada (12)
MedTech (12)
sfda (12)
DoC (12)
TGA (11)
Singapore (11)
regulations (11)
saudi arabia (11)
Digital (11)
RegDes (11)
Regulatory Approval (11)
USA (10)
510k (10)
Clinical Trials (10)
ivd (10)
MDCG (10)
AI Medical Device Regulations (10)
Food and Drug Administration (10)
cdrh (9)
fda medical devices (9)
Canada (8)
Public health (8)
Asia (7)
EU MDR (7)
hsa (7)
software (7)
Regulatory Authority (6)
European Union (6)
medical (6)
IMDRF (6)
requirements (6)
European (6)
Australia (5)
China (5)
Anvisa (5)
Brazil (5)
Product Registration (5)
NMPA (5)
artg (5)
medicines (5)
USFDA (5)
Clinical Research (5)
RegDesk (74)
Medical Device (72)
FDA (58)
united states (30)
healthcare (29)
Healthcare Products (28)
mdr (27)
RIMS (26)
FDA guidance (25)
Regulatory Compliance (24)
regulatory (23)
guidance (23)
guidance RegDesk (23)
regulation (22)
medical device regulations (21)
Medical Devices RegDesk (19)
regulatory affairs (17)
United States of America (17)
medical software (16)
Europe (15)
medical device regulation (15)
IVDR (14)
North America (14)
medical device registration (13)
Global Manufacturers (13)
Medical Technologies (13)
EU (12)
health canada (12)
MedTech (12)
sfda (12)
DoC (12)
TGA (11)
Singapore (11)
regulations (11)
saudi arabia (11)
Digital (11)
RegDes (11)
Regulatory Approval (11)
USA (10)
510k (10)
Clinical Trials (10)
ivd (10)
MDCG (10)
AI Medical Device Regulations (10)
Food and Drug Administration (10)
cdrh (9)
fda medical devices (9)
Canada (8)
Public health (8)
Asia (7)
EU MDR (7)
hsa (7)
software (7)
Regulatory Authority (6)
European Union (6)
medical (6)
IMDRF (6)
requirements (6)
European (6)
Australia (5)
China (5)
Anvisa (5)
Brazil (5)
Product Registration (5)
NMPA (5)
artg (5)
medicines (5)
USFDA (5)
Clinical Research (5)