This chart lists the pharmaceutical, medical and related regulatory authorities of MENA regulations countries along with links to their relevant websites. 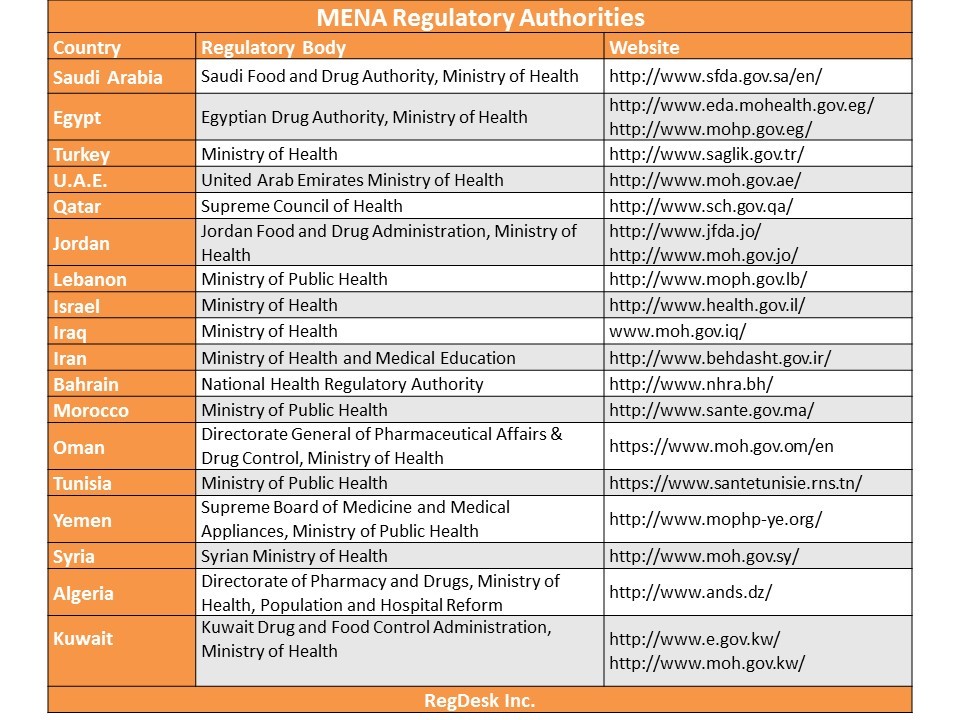
Categories
Categories
- ACTD (1)
- Africa (4)
- AI (1)
- AIaMD (2)
- ANMAT (1)
- ANVISA (5)
- Argentina (1)
- ARTG (2)
- Asia (62)
- Singapore (30)
- Australia (46)
- Australia and Oceania (16)
- Austria (1)
- Bahrain (6)
- BASG (1)
- BfArM (18)
- Brazil (10)
- ANVISA (1)
- Canada (38)
- CBRN (1)
- CDC (1)
- CDRH (11)
- CDSCO (8)
- China (4)
- CHMP (1)
- CLIA (1)
- Consultant Post (1)
- COVID-19 (3)
- Czech Republic (1)
- Denmark (1)
- DHSC (1)
- DRAP (27)
- DRCPFA (1)
- EDA (21)
- Egypt (21)
- Ethiopia (4)
- EU (51)
- EU MDR/IVDR (27)
- EUA (1)
- Europe (107)
- European Commission (3)
- European Union (11)
- FDA (267)
- Germany (18)
- GMDN (1)
- GMLP (1)
- Great Britain (15)
- GRTPNZ (3)
- GSPR (1)
- Guatemala (1)
- Health Canada (20)
- Healthcare (2)
- HIE (1)
- Hong Kong (2)
- HPRA (7)
- HSA (29)
- IMDRF (4)
- India (8)
- Ireland (7)
- ISO Standards (1)
- Kazakhstan (6)
- Korea (1)
- KSA (1)
- Latvia (1)
- LRN (1)
- Malaysia (14)
- MDA (9)
- MDACs (2)
- MDCG (64)
- MDR (5)
- MDSAP (2)
- Medical Device Regulation Resources (1)
- Medical Devices (26)
- Medsafe (8)
- Mexico (1)
- MFDS (1)
- MHRA (26)
- MoU (2)
- NATA (2)
- New Zealand (8)
- NHRA (6)
- NMPA (2)
- North America (112)
- Northern Ireland (1)
- NPAAC (3)
- Pakistan (27)
- PDUFA (3)
- Peru (1)
- PHEs (1)
- Philippines (3)
- Poland (1)
- QMS (2)
- RCPA (1)
- RegDesk News/Info (28)
- RIMS (23)
- Russia (1)
- SAHPRA (5)
- SaMD (3)
- Saudi Arabia (23)
- Serbia (1)
- SFDA (11)
- SFHS (2)
- South Africa (6)
- South America (11)
- South Korea (2)
- State Agency of Medicines of Latvia (1)
- Swissmedic (10)
- switzerland (10)
- Taiwan (2)
- Tanzania (6)
- TGA (58)
- TMDA (6)
- TMMDA (1)
- Turkey (6)
- Turkish national regulating authority (1)
- Turkish Regulatory Authority (1)
- UK (13)
- Uncategorized (76)
- United Kingdom (18)
- United States (272)
- URRA (4)
- USA (4)
- USFDA (10)
Tags
medical devices (407)
Medical Device (167)
FDA (156)
Healthcare Products (128)
RegDesk (124)
FDA guidance (87)
RIMS (85)
united states (72)
mdr (71)
guidance (69)
regulatory (56)
regulation (53)
healthcare (50)
medical device regulation (44)
TGA (43)
Regulatory Compliance (42)
medical software (41)
guidance RegDesk (40)
medical device regulations (39)
regulatory affairs (38)
MDCG (38)
Singapore (35)
health canada (34)
Medical Devices RegDesk (32)
United States of America (31)
IVDR (31)
Clinical Trials (30)
Regulatory Approval (30)
Europe (29)
Public health (29)
EU (28)
regulations (28)
hsa (28)
ivd (28)
510k (26)
North America (26)
USA (24)
cdrh (24)
Canada (23)
sfda (23)
Clinical Research (23)
saudi arabia (22)
Patient safety (20)
Australia (19)
USFDA (19)
Therapeutic Goods (19)
medicines (18)
IVDs (17)
requirements (17)
mhra (16)
fda medical devices (16)
artg (16)
RegDes (16)
Food and Drug Administration (16)
medical device registration (15)
MedTech (15)
software (15)
Digital (15)
FD&C Act (15)
Asia (14)
Digital Health (14)
medical (14)
classification (14)
guidelines (14)
medical device grouping (14)
NPC (14)
Malaysia (13)
regulatory guidance (13)
Product Registration (13)
conformity assessment (13)
DRAP (13)
mda (12)
IMDRF (12)
medical products (12)
Device Classification (12)
GCP (12)
National Pharmacovigilance System (12)
Global Manufacturers (12)
Medical Technologies (12)
Regulatory Authority (11)
Labeling (11)
notified bodies (11)
guidances (11)
BfArM (11)
Austalia (11)
DoC (11)
Member States (11)
European Union (10)
EU MDR (10)
Germany (10)
clinical investigations (10)
Recalls (10)
Clinical Investigation (10)
AI Medical Device Regulations (10)
FDA approval (10)
Pakistan (9)
combination products (9)
medicinal products (9)
COVID-19 (9)
Product Recalls (9)
Anvisa (8)
Brazil (8)
UDI (8)
regulatory requirements (8)
safety (8)
Q-Submission (8)
Clinical Studies (8)
Regulatory Intelligence (8)
TPLC (8)
PPI (8)
Radiation Control Regulations (8)
Registration (7)
Kazakhstan (7)
South Asia (7)
Medical Device Industry (7)
Cybersecurity (7)
EUMDR (7)
risk management (7)
European (7)
CABs (7)
overview (7)
regdesk medical device (7)
qms (7)
healthcare technology (7)
Medical Device Improvement (7)
Life Cycle Approach (7)
Software Medical Devices (7)
Medicines Act (7)
IVD Medical Devices (7)
Biological Products (7)
Turkey (6)
Regulatory News (6)
US (6)
ISO (6)
medical device classification (6)
SaMD (6)
HPRA (6)
clinical trial (6)
IDE (6)
clinical evaluation (6)
Application process (6)
Establishment Licensing (6)
Adverse Events (6)
Good Clinical Practices (6)
Recall PRocedure (6)
FD&C (6)
Specific Aspects (6)
Pharmacy Profession Law (6)
Animal Health (6)
Waste Management (6)
Public Safety (6)
Health Protection (6)
Healthcare System (6)
JAT (6)
Excluded Software (6)
Good Review Practices (6)
Regulatory Bodies (6)
Preclinical Testing (6)
Testing Requirements (6)
Regulatory Legislation (5)
China (5)
Egypt (5)
Pharma (5)
Pharmacovigilance (5)
TGA news (5)
Medical Device regulatory (5)
health products (5)
NMPA (5)
devices (5)
risk classification (5)
medical device submissions (5)
third party review (5)
dental (5)
conformity assessment bodies (5)
NDDA (5)
MRDD (5)
sterilization (5)
Health Sciences Authority (5)
Medical Device Labeling (5)
Southeast Asia (5)
sahpra (5)
21 CFR (5)
Neonatal Product Development (5)
Fast-Track Process (5)
Healthcare Innovation (5)
GSPR (5)
Fast Track Approval (5)
Clinical study (5)
Real World Evidence (5)
In-House IVDs (5)
Diversity (5)
Patient Preference (5)
Chemical Analysis (5)
Biocompatibility Assessment (5)
Chemical Characterization (5)
Electronic Records (5)
Electronic Systems (5)
Electronic Signatures (5)
GRevP (5)
PMS (5)
X-Ray Equipment (5)
IEC (5)
Personalized Devices (5)
RDC (5)
Medical Device (167)
FDA (156)
Healthcare Products (128)
RegDesk (124)
FDA guidance (87)
RIMS (85)
united states (72)
mdr (71)
guidance (69)
regulatory (56)
regulation (53)
healthcare (50)
medical device regulation (44)
TGA (43)
Regulatory Compliance (42)
medical software (41)
guidance RegDesk (40)
medical device regulations (39)
regulatory affairs (38)
MDCG (38)
Singapore (35)
health canada (34)
Medical Devices RegDesk (32)
United States of America (31)
IVDR (31)
Clinical Trials (30)
Regulatory Approval (30)
Europe (29)
Public health (29)
EU (28)
regulations (28)
hsa (28)
ivd (28)
510k (26)
North America (26)
USA (24)
cdrh (24)
Canada (23)
sfda (23)
Clinical Research (23)
saudi arabia (22)
Patient safety (20)
Australia (19)
USFDA (19)
Therapeutic Goods (19)
medicines (18)
IVDs (17)
requirements (17)
mhra (16)
fda medical devices (16)
artg (16)
RegDes (16)
Food and Drug Administration (16)
medical device registration (15)
MedTech (15)
software (15)
Digital (15)
FD&C Act (15)
Asia (14)
Digital Health (14)
medical (14)
classification (14)
guidelines (14)
medical device grouping (14)
NPC (14)
Malaysia (13)
regulatory guidance (13)
Product Registration (13)
conformity assessment (13)
DRAP (13)
mda (12)
IMDRF (12)
medical products (12)
Device Classification (12)
GCP (12)
National Pharmacovigilance System (12)
Global Manufacturers (12)
Medical Technologies (12)
Regulatory Authority (11)
Labeling (11)
notified bodies (11)
guidances (11)
BfArM (11)
Austalia (11)
DoC (11)
Member States (11)
European Union (10)
EU MDR (10)
Germany (10)
clinical investigations (10)
Recalls (10)
Clinical Investigation (10)
AI Medical Device Regulations (10)
FDA approval (10)
Pakistan (9)
combination products (9)
medicinal products (9)
COVID-19 (9)
Product Recalls (9)
Anvisa (8)
Brazil (8)
UDI (8)
regulatory requirements (8)
safety (8)
Q-Submission (8)
Clinical Studies (8)
Regulatory Intelligence (8)
TPLC (8)
PPI (8)
Radiation Control Regulations (8)
Registration (7)
Kazakhstan (7)
South Asia (7)
Medical Device Industry (7)
Cybersecurity (7)
EUMDR (7)
risk management (7)
European (7)
CABs (7)
overview (7)
regdesk medical device (7)
qms (7)
healthcare technology (7)
Medical Device Improvement (7)
Life Cycle Approach (7)
Software Medical Devices (7)
Medicines Act (7)
IVD Medical Devices (7)
Biological Products (7)
Turkey (6)
Regulatory News (6)
US (6)
ISO (6)
medical device classification (6)
SaMD (6)
HPRA (6)
clinical trial (6)
IDE (6)
clinical evaluation (6)
Application process (6)
Establishment Licensing (6)
Adverse Events (6)
Good Clinical Practices (6)
Recall PRocedure (6)
FD&C (6)
Specific Aspects (6)
Pharmacy Profession Law (6)
Animal Health (6)
Waste Management (6)
Public Safety (6)
Health Protection (6)
Healthcare System (6)
JAT (6)
Excluded Software (6)
Good Review Practices (6)
Regulatory Bodies (6)
Preclinical Testing (6)
Testing Requirements (6)
Regulatory Legislation (5)
China (5)
Egypt (5)
Pharma (5)
Pharmacovigilance (5)
TGA news (5)
Medical Device regulatory (5)
health products (5)
NMPA (5)
devices (5)
risk classification (5)
medical device submissions (5)
third party review (5)
dental (5)
conformity assessment bodies (5)
NDDA (5)
MRDD (5)
sterilization (5)
Health Sciences Authority (5)
Medical Device Labeling (5)
Southeast Asia (5)
sahpra (5)
21 CFR (5)
Neonatal Product Development (5)
Fast-Track Process (5)
Healthcare Innovation (5)
GSPR (5)
Fast Track Approval (5)
Clinical study (5)
Real World Evidence (5)
In-House IVDs (5)
Diversity (5)
Patient Preference (5)
Chemical Analysis (5)
Biocompatibility Assessment (5)
Chemical Characterization (5)
Electronic Records (5)
Electronic Systems (5)
Electronic Signatures (5)
GRevP (5)
PMS (5)
X-Ray Equipment (5)
IEC (5)
Personalized Devices (5)
RDC (5)