General Context
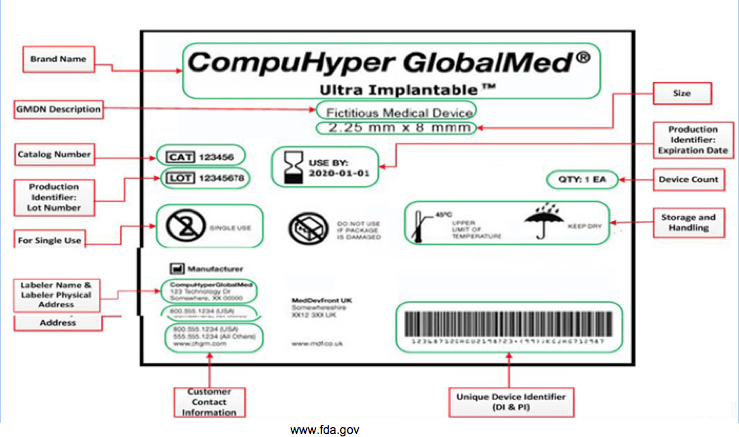
- UDI is a Unique Device Identifier in the form of a unique number or alphanumeric code. It consists of plain text and AIDC (only readable via machine)
- Issued by an FDA-accredited issuing agency. UDI is granted for 3 years and can be renewed upon submission and FDA approval of a renewal application.
- UDI should contain:
- Device Identifier (DI) – Model and the labeler of the device. DI is mandatory so all UDIs must contain DI.
- Production Identifier (PI) – Portion of the UDI should identify one or more of the following attributes when the UDI is placed on the label of the device (conditional):
- Lot or batch number
- Serial number
- Expiration date
- Manufacturing date
- Distinct Identification Code (For Human Cell and Tissue Product)
- Placed at the bottom right of the device label.
- The Global Unique Device Identification Database (GUDID) ONLY stores DI and not PI. However, there are PI flags to indicate which PI attributes are on the device label.
Steps to Establish UDI
- Hire an accredited Issuing Agency that develops the unique labeler code for use in UDIs.
- Place the UDI on the label and package and for some devices, on the device itself with Plain Text and Machine Readable AIDC.
- Enter the data in the UDI Database (GUDID).
Important Dates
| Due Date | Device Class |
| September 24, 2014 | Class III, Class III stand alone software, Devices licensed under the PHS Act |
| September 24, 2015 | Implantable, life-supporting and life sustaining devices including stand alone software |
| September 24, 2016 | All Class II devices, direct marking for Class III devices and devices licensed under the PHS Act for certain intended uses |
| September 24, 2018 | Class I devices, devices not classified as Class I, II or III, direct marking of class II devices for certain intended uses |
| September 24, 2010 | Direct Marking of Class I devices, devices not classified into Class I, II or III for certain intended uses |

